Design of EMBLEM
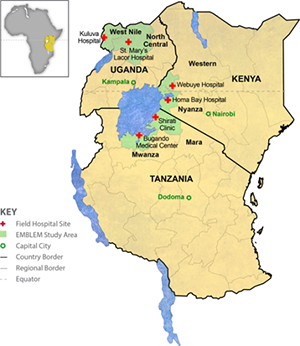
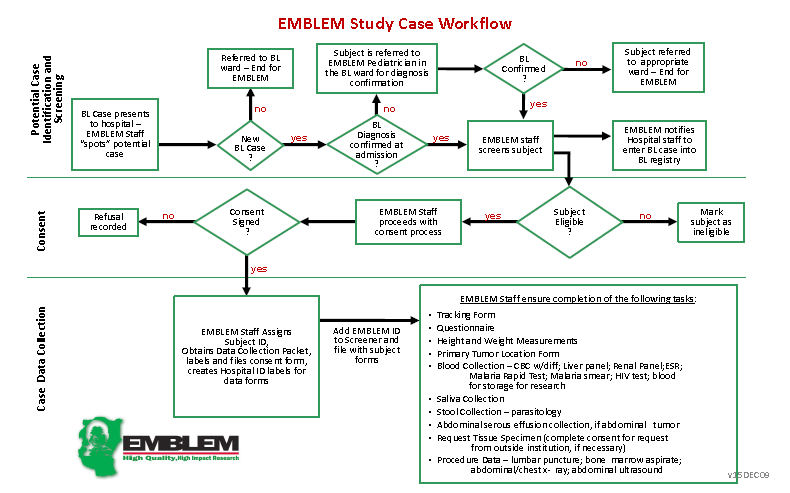
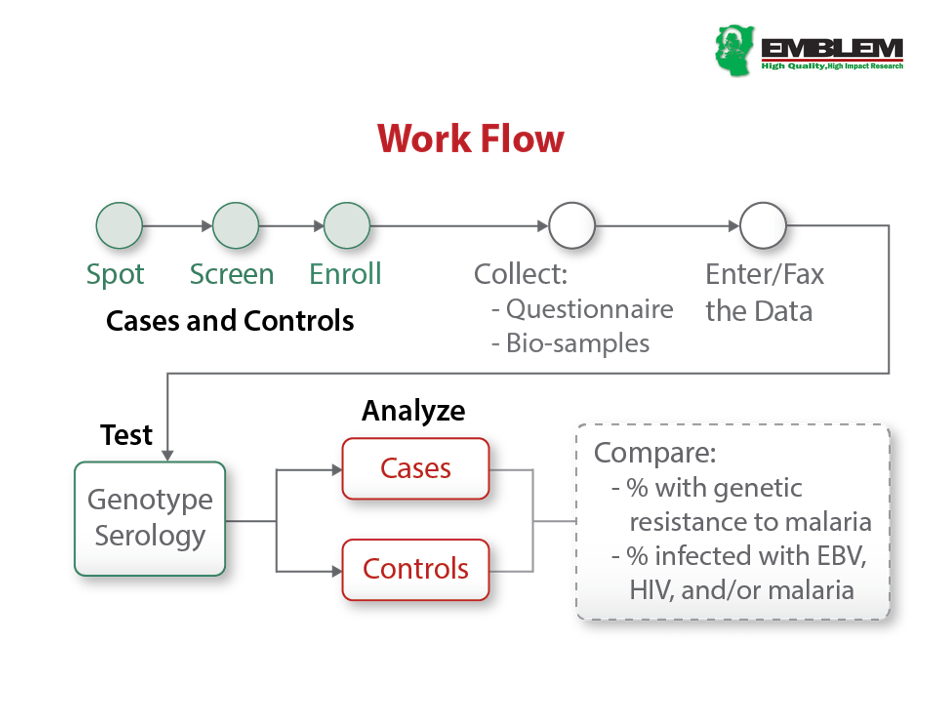
EMBLEM was a population-based case-control study conducted during 2010-2016 in six regions in East Africa, where malaria transmission is holoendemic and year-round (see figure). Participants who were usual residents (> four months prior to enrollment) of the study area and aged 0-15 were eligible. Cases were defined based on histological and/or clinical features, imaging, and laboratory results compatible with an endemic Burkitt lymphoma (eBL) diagnosis (see Study Case Workflow below). Controls were randomly selected from 300 villages (100 per country) as a representative sample of the source population for cases.
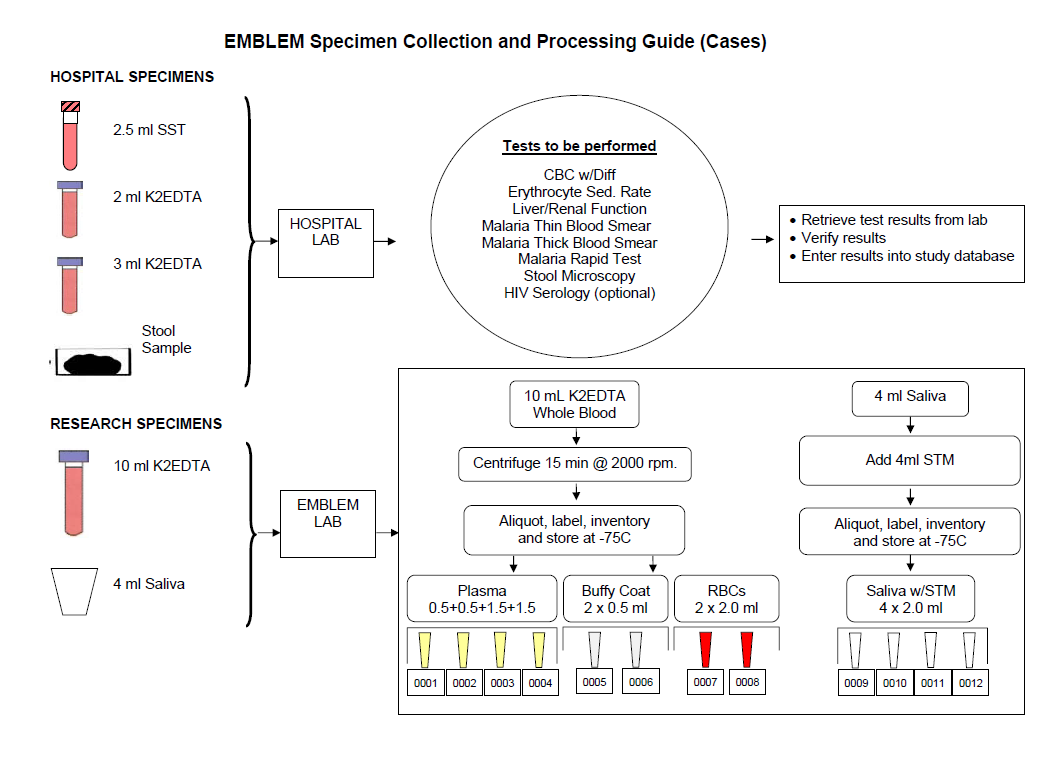
Standardized protocols were used to capture research data, including age, sex, parental and household exposures, history of inpatient and outpatient malaria treatment, malaria and other fever, and use of indoor residual spraying (IRS) in the house, insecticides, and mosquito bed nets. Both cases and controls provided research blood, saliva, and from a proportion of cases, tumor tissues samples, which were processed into aliquots and stored at -800C (see Samples Workflow below).
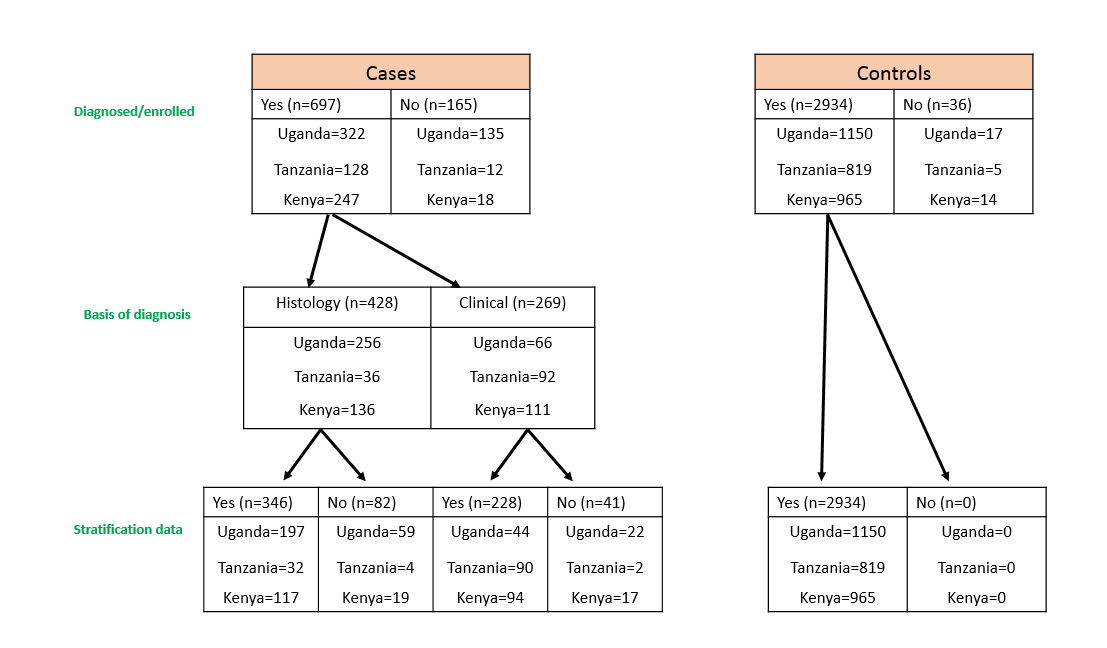
EMBLEM accrued 800 cases and 2900 matched population controls (see Accrual in EMBLEM below), collecting some ~50,000 vials of samples, of which ~25,000 have been shipped to The National Cancer Institute (NCI) for further study.